Pharmaceuticals
MINIMUM LIQUID DISCHARGE IN AN EXTREMELY LIMITED FOOTPRINT
Only Gradiant was able to provide a Minimum Liquid Discharge solution to meet GSK’s demanding performance specification within an extremely limited existing footprint. Engineering a bespoke Carrier Gas Extraction design where others had failed.
Fast Facts
Location: Singapore
End User: GlaxoSmithKline (GSK)
Solution: Minimum Liquid
Industry: Pharmaceuticals
Feedwater Source: Wastewater from Medicine Production
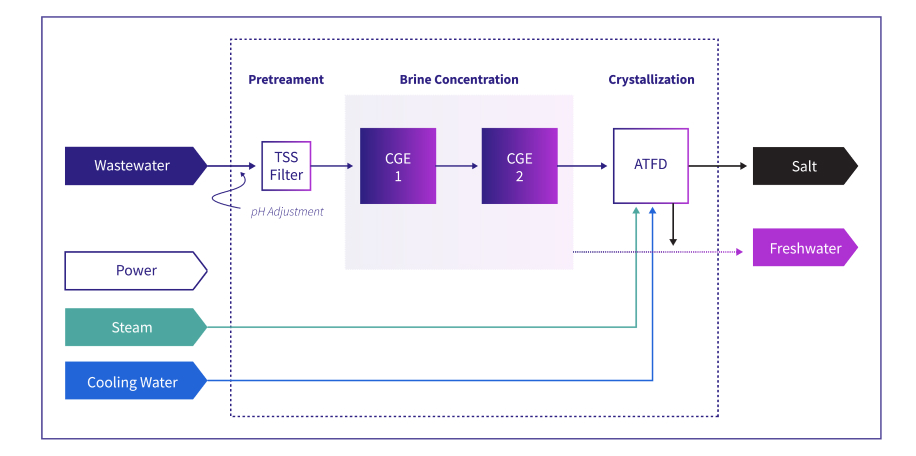
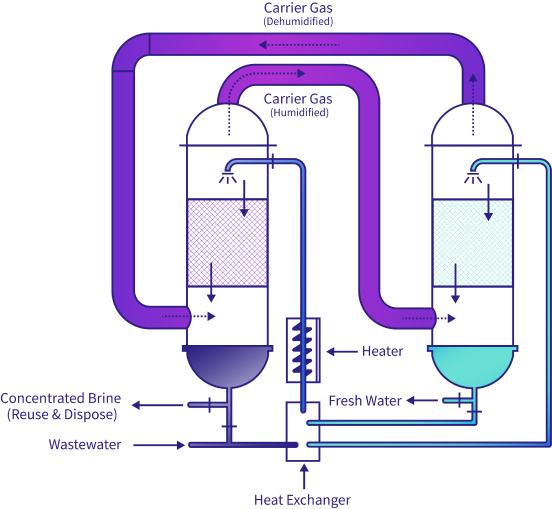
Technology: Carrier Gas Extraction (CGE), Agitated Thin Film Dryer (ATFD)
System Configuration: 2 x 50% CGE and 100% ATFD
System Capacity: 288 m³/day
System Recovery: 98%
Online Date: June 2022
Delivery Model: Design-Build (DB)
The Challenge
GlaxoSmithKline (GSK) is a global pharmaceutical and biotech company, and a leading manufacturer of amoxicillin. GSK’s antibiotics manufacturing plant in Singapore produced wastewaters containing organic solvents and unrecovered amoxicillin products, which were restricting overall manufacturing yields and waste disposal. The primary challenge was to identify a compact process solution that could sustainably treat high COD, TDS, and Chlorides feedwater. All within an extremely challenging limited footprint available at the brownfield site.
GSK had been unsuccessful sourcing a credible solution — enter Gradiant.
The Solution
A bespoke version of our Carrier Gas Extraction technology — patented and award winning. The dedicated project delivery team deployed bench-scale lab testing to demonstrate proof of concept and the proven cost advantages of using CGE. Quantified results were complimented by in-house unique design innovation for the design-build project phase of this MLD facility – creating savings of 35% in CAPEX and 50% in OPEX relative to competitors.
In an unprecedented deployment, Gradiant’s engineers designed the CGE technology as two 32-meter towers in order to accommodate the existing facility’s very limited footprint. The CGE technology has been proven to help other clients achieve 20x brine concentration, and when combined with ATFD, reduce overall disposal volumes by over 98%, while lowering effluent COD and TDS concentrations. The ZLD process scheme feeds the remaining concentrated brine to an Agitated Thin Film Dryer to achieve >80% purity solids cake.
The Benefits
Gradiant’s solution proved to be superior on a technical and economic basis to address brine concentration and minimum liquid discharge — overall disposal volumes were reduced by over 98%, while lowering effluent COD and TDS concentrations. By implementing the solution, our client GSK could focus on providing the world supply of amoxicillin and other key medicines. Exceeding expectation by providing savings of up to 35% and 50% in CAPEX and OPEX compared to competitor technologies.
Following the project’s successful deployment, Gradiant is creating opportunities for other pharmaceutical brand owners to bring sustainability into their operations and solve their unique manufacturing challenges – to ensure their focus remains on producing life saving medicines and cures for the global population.
Impact Stats
98%
Reduction in Disposal Volume
ZERO
Liquid Discharge
50%
Up to 50% Less Thermal Energy than Multi-Effect Evaporation and Distillation(MEE, MED) systems
50%
Up to 50% CAPEX and OPEX Savings versus the Competitors
50%
More than 50% Energy Savings compared to Mechanical Vapor Compression (MVC) evaporation
Figures & Images
GSK Process Flow Diagram
Carrier Gas Extraction Process Flow Schematic
Legal Disclaimer
This document is for general information only. No warranty or guarantee whatsoever is given or implied and Gradiant is not bound by or liable for or by the information contained herein. Customer has the sole responsibility to determine whether the information in this document are appropriate for Customer’s use, including without limitation actual site, geographical, and plant conditions, specifications, requirements, disposal, applicable laws and regulations. This document is the intellectual property of Gradiant, including but not limited to any patent or trademark contained in this document. Distribution of this document is not and does not imply any transfer of Gradiant’s intellectual property.
Gradiant, the Gradiant Logo, and all trade and service marks denoted with ™ or ® are owned by affiliates of Gradiant Corporation unless otherwise noted. © 2024 Gradiant.






